DQ SERUM
Contact and commercial agreements with meat producers of great importance at the national level opened up the possibility of having the raw material of excellent quality to venture into the production of fetal bovine serum, guaranteeing the traceability of the raw material and compliance with all the standards established by Tet (aseptic fetal bovine blood collection system in sterile plastic bags with official ante and post mortem inspection of the animal).
The fetal bovine serum obtained contains a large number of macromolecular and nutritional factors for cell growth, and in turn does not contain derivatives that interfere with the proper regulation of gene expression controlled by TRE (ability of a gene to produce a biologically active protein) and control of vaccines, biotechnological drugs for human and animal consumption.
The quality of Doquimta fetal bovine serum is guaranteed by the traceability of the raw material, its production process, by compliance with levels of growth factors, endotoxins, osmolality, its low protein content makes it have an effect of no lyse cells in culture and not interfere with immunoassays; as well as their high individual performance in cell culture.
Our fetal bovine serum has a wide range of applications in the areas of research, manufacturing and control of vaccines, biotechnological drugs for human and animal consumption.

PRESENTATIONS

LIQUID
DOQUIMTA fetal bovine serum is manufactured in Mexico.
Storage conditions: Store at -20° c.
Duration: 1 year under storage conditions.
Certificate of analysis: Number 32313, issued by the laboratory of immunological specialties (lei), with health license 09 007 130001.
This fetal bovine serum has been tested and found to meet the following specifications:
| Appearance: | Clear amber liquid |
| Odor: | Odorless |
| Bacteria and fungi: | Not detected |
| Mycoplasma DNA: | Not detected |
| Bav1 virus test: | Not detected |
| Test bav5: | Not detected |
| pH at room temperature: | 6.90 |
| Osmolality 260-350 mOsm / kg | 348 mOsm/kg |
| Endotoxin ≤ 10.0 EU/ml: | 0.0043 EU/ml |
| Hemoglobin ≤ 30.0mg/ml: | 19 mg/ml |
| Electrophoretic pattern: | Normal |
| Immunoglobulin: | lgg: 12.9825 mg/ml |
| Proteins 30-45 mg/ml: | 32 mg/ml |
| Solubility in water: | 0.05 g/l at -20 °C |
| Explosive properties: | Not classified as explosive |
The immediate disposal of doquimta fetal bovine serum is 20 liters per week
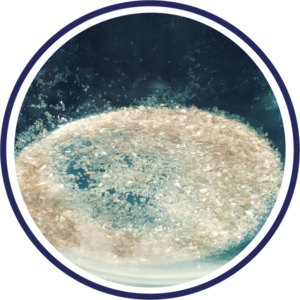
CRYSTALLIZED
DOQUIMTA fetal bovine serum is manufactured in Mexico.
Product description: Crystallized without cells, fibrin or coagulation factors containing a large number of macromolecular and nutritional factors essential for cell growth.
Aplications: It is excellent for use as a nutrient in culture media for the production of antibiotics, toxins, enzymes and others biological products. It is widely used in industry pharmaceutical, veterinary, media culture for diagnosis, research, manufacturing, vaccine control, drugs biotechnological for human consumption and animal.
Physical characteristics: Solid in crystals.
Presentation: 5, 10, 20, 50 gr.
Storage conditions: Without refrigeration on the shelf and no more than 30 °C at room temperature
Duration: 1 year under storage conditions.
Certificate of analysis: Number 32313, issued by the laboratory of immunological specialties (lei), with health license 09 007 130001.
Documents: Certificate of analysis and safety data sheet.
This fetal bovine serum has been tested and found to meet the following specifications:
| Appearance: | Amber colored crystallized solid |
| Electrophoretic pattern: |
Regular. |
| Bacteria and fungi: | Not detected |
| Mycoplasma DNA: | Not detected |
| Mycoplasma Test: | Negative (USP 42, NF37, Ed 2017). |
| Virus BAV 1 test: | Not detected |
| BAV Test 5: | Not detected |
| pH at room temperature: | 6.90 in 10% dissolution |
| Osmolality 260-350 mOsm /kg | 348 mOsm/kg |
| Endotoxin ≤ 10.0 EU/ml: | 0.0043 EU/ml |
| Endotoxin test: |
>=0 A <=50 EU/ml (USP 40, NF35, Ed 2017). |
| Hemoglobin ≤ 30.0mg/ml: | 19 mg/ml |
| Hemoglobin: | >=0 A <=30 mg (USP 40, NF35, Ed 2017) |
| Electrophoretic pattern: | Normal |
| Immunoglobulin: | 12.9825 mg/ml |
| Immunoglobulins: | <=15 mg/ml (by gel filtration IMFA-015-18). |
| Proteins 30-45 mg/ml: | 32 mg/ml |
| Albumin: | 1.8 g/al |
| Alkaline phosphatases: | 185 U/I |
| Glucose: | 98 mg/dl |
| pH: | >=6.9 A <= 7.8. (USP 791). |
| Total Proteins: | >=3.0 A <=4.0 G/DI. (BIURET METHOD). |
| Fluorescence Antibody Virus Assays (9CFR-113.53). |
|
| -VT- BLUTONGUE VIRUS FA: NEGATIVE -VT- CYTOPATHOGENIC AGENTS: NEGATIVE -VT- BOVINE ADENOVIRUS FA: NEGATIVE -VT- HEMADSORBING AGENTS: NEGATIVE -VT- BOVINE PARVOVIRUS FA: NEGATIVE -VT- RABIES VIRUS FA: NEGATIVE -VT- BRSV FLUORESCENT ANTIBODY: NEGATIVE -VT- REOVIRUS FA: NEGATIVE |
|
The immediate availability of Doquimta fetal bovine serum is 5 kg per week.
PROCESSING AND FINISHING
El The serum never comes into contact with the parts that require washing or CIP/SIP (Clean In Place / Steam In Place), removing the potential for cross contamination with other products or cleaning products.
All batches go through triple filtration of 0.1 microns, a process that has been validated to kill mycoplasma.
The serum crystals are aseptically dispensed into ultraviolet sterilized bottles in a HEPA filtered Class 100.
DISSOLUTION
Take 5.25 or 50 gr and dissolve them in 100, 500 or 1000 ml of depyrogenated water with the help of a vortex for 30 minutes to get 100, 500 or 1000 ml of liquid serum.
BATCH VARIATION
Doquimta performs cell culture and MTT assay on each batch to ensure its growth promoting ability and not cytotoxicity.
Each end user is recommended to test the batch on their particular cell line in order to determine its suitability for the intended use.
TRACEABILITY
The material used in the production of SFB of Doquimta is collected in traces coded by SAGARPA or other government entity pertinent, being inspected and approved.
A Certificate of Origin is available for each lot.
Doquimta is capable of tracking any batch of SFB from any slaughterhouse in the country.
SAFETY DATA SHEET
According to Regulation (EC) No. 1907/2006 – Input for medical uses issued by COFEPRIS
| SECTION 1. IDENTIFICATION OF THE SUBSTANCE OR MIXTURE AND OF THE COMPANY OR UNDERTAKING | |
| 1.1 PRODUCT IDENTIFIER | |
| Lot No.: | |
| Denomination: | Bovine Serum for Biochemical Purposes |
| Record of the annual tonnage produced: | A registration number for this substance is not available, as that the substance or its use is exempt from registration; according to the Article 2 of REACH Regulation (EC) No. 1097/2006, the Annual tonnage does not require registration or such registration is planned for a later date. |
| Storage No.: | |
| 1.2 IDENTIFIED RELEVANT USES OF THE SUBSTANCE OR MIXTURE | |
| Identified uses: | Biochemical research and analysis For additional information on uses refer to the email of Doquimta Laboratory (laboratorio@doquimta.com) |
| 1.3 DETAILS OF THE SUPPLIER OR THE PERSON RESPONSIBLE FOR THE SAFETY DATA SHEET | |
| Company: | Doquimta Laboratory |
| Department responsible: | laboratorio@doquimta.com |
| Address: | Margaritas #102 Fraccionamiento Las Palomas, Mineral de la Reforma, Hgo. |
| Telephone: | (01 771) 791 27 86, Ext. 4300 |
| Emergency phone: | Instituto Nacional de Toxicología, México, Tel: 5556762767 |
| SECTION 2. HAZARD IDENTIFICATION | |
| 2.1 CLASSIFICATION OF THE SUBSTANCE OR MIXTURE | |
| This substance is not classified as dangerous according to the EMA (EUROPEAN MEDICINES AGENCY) legislation of the EUROPEAN UNION, the FDA (FOOD AND DRUG ADMINISTRATION) of the USA and the COFEPRIS (COMISIÓN FEDERAL PARA LA PROTECCIÓN CONTRA RIESGOS SANITARIOS – FEDERAL COMMISSION FOR THE PROTECTION AGAINST HEALTH RISKS) of MEXICO. | |
| Item No.: | 112018 |
| Product Name: | Fetal Bovine Serum for Biochemical Purposes |
| 2.2 LABEL ELEMENTS | |
| It is not a hazardous substance or mixture according to Regulation (EC) No. 1272/2008. | |
| SECTION 3. COMPOSITION/ INFORMATION ON COMPONENTS | |
| 3.1 SUBSTANCE | |
| Certificate issued: | 292-322-5 |
| Comments: No ingredient is considered hazardous according to Regulation (EC) No. 1907/2006 – Input for medical uses issued by COFEPRIS. | |
| 3.2 MIXING | |
| Not applicable. | |
| SECTION 4. FIRST AID | |
| 4.1 DESCRIPTION OF FIRST AID | |
| a) After inhalation: Take fresh air (Does not produce any side effects). b) In case of skin contact: Immediately remove all contaminated clothing. c) Rinse the skin with plenty of water. d) Contact with eyes: Wash with plenty of water. e) In case of ingestion: Drink plenty of water (maximum 2 glasses). f) In case of discomfort: Consult the doctor. |
|
| 4.2 MAIN SYMPTOMS AND EFFECTS, ACUTE AND DELAYED | |
| No symptoms of toxicity were detected. | |
| SECTION 5. MEASURES IN CASE OF ACCIDENTAL SPILLAGE | |
| 5.1 PERSONAL PRECAUTIONS, PROTECTIVE EQUIPMENT AND EMERGENCY PROCEDURES | |
| Instructions for personnel who are not part of the emergency services: a) Avoid inhalation of dust. b) Evacuate the danger area, respect emergency procedures, consult with experts. Tips for emergency personnel: c) Wear protective equipment. |
|
| 5.2 ENVIRONMENTAL PRECAUTIONS | |
| It does not emit any type of toxic or polluting waste. | |
| 5.3 CONTAINMENT AND CLEANING METHODS AND MATERIAL | |
| a) Dry collect and proceed to the disposal of waste. b) Rinse the affected surface with water. c) Avoid the formation of dust. |
|
| SECTION 6. HANDLING AND STORAGE | |
| 6.1 PRECAUTIONS FOR SAFE HANDLING | |
| Prior knowledge is recommended for safe handling and to observe the indications on the label to know the Handling procedure. Hygiene measures: a) Use the appropriate protective equipment. b) Wash hands with soap and water at the end of work. |
|
| 6.2 SECURE STORAGE CONDITIONS, INCLUDING POSSIBLE INCOMPATIBILITIES | |
| Storage conditions a) Store on the shelf at room temperature not exceeding 30 °C. |
|
| SECTION 7. EXPOSURE CONTROLS/INDIVIDUAL PROTECTION | |
| 7.1 CONTROL PARAMETERS | |
| It does not contain substances with occupational exposure limit values. | |
| 7.2 EXPOSURE CONTROLS | |
| a) Engineering measures. b) Technical measures and observance of appropriate working methods take precedence over the use of protective equipment personnel |
|
INDIVIDUAL PROTECTION MEASURES
PROTECTIVE EQUIPMENT
The types of body protection aids should be chosen specifically according to the job depending on the concentration and amount of the dangerous substance. The stability of protective media against chemicals should be clarified with the supplier.

Eyes and face protection:
Safety glasses.

Body protection:
Use of safety gloves.
Use of laboratory overalls.
Use of special shoes.

Submersion:
Glove material: Nitrile rubber.
Glove thickness: 0.11 mm.
Penetration time: Greater than 480 min.

Splashes:
Glove material: Nitrile rubber.
Glove thickness: 0.11 mm.
Penetration time: Greater than 480 min.
The indicated protective gloves must comply with the specifications of Directive 89/686/EEC and its resulting standard EN374, for example KCL 741 Dermatril® L (Immersion), KCL 741 (Splashes) and Dermatril® L (Splashes).